Gene therapy for Haemophilia A and B has been under development for many years. In recent years, the majority of clinical trials have been based on the use of an adeno associated viral (AAV) vector for delivery of the gene therapy genetic material to the hepatocytes in the liver. In reality, this is gene addition therapy as the vast majority of the genetic material delivered to the liver cells does not integrate into your existing DNA but sits separately within the cell and hopefully produces either factor VIII or factor IX.
There are currently 16 gene therapy trials for haemophilia in phase 1 or 2 and 5 in phase 3. With AAV based haemophilia gene therapies, the genetic material is contained within an AAV viral capsid. The capacity of this capsid is limited and therefore all of the factor VIII gene therapy clinical trials use a beta domain deleted factor VIII gene. For factor IX, it was discovered that there was a high activity variant called the padua variant which can give much higher factor IX expression. This factor IX Padua variant is now used in many of the factor IX gene therapy clinical trials.
The first hemophilia gene therapy to be licensed was a factor VIII gene therapy, Roctavian, which was licensed by the European Medicines Agency (EMA) in September 2022.The first factor IX gene therapy, Hemgenix, was licensed by the FDA in the USA on November 22, 2022. It is anticipated that this gene therapy will also be licensed by the EMA by quarter 1 of 2023.
Currently, gene therapy is an irreversible one-off therapy which cannot be repeated due to the fact that the individual will develop antibodies to the AAV vector once they have received the therapy, thereby preventing them being re-dosed. Eligibility for gene therapy will be for those with severe haemophilia over the age of 18, without a history of inhibitors, without serious liver disease and without preexisting antibodies to the AAV vector. It is anticipated that 40% to 50% of people with severe factor VIII deficiency will be theoretically eligible for gene therapy and 40% to 60% of those with severe factor IX deficiency will be theoretically eligible (due to the ability to dose those with pre-existing AV antibodies for one of the factor IX gene therapies.)
If the haemophilia gene therapies post licensing are reimbursed, there will be a requirement for a significant education programme for the gene therapy to be undertaken by the individual before they decide if gene therapy is an option for them. An education program will be rolled out by the Irish haemophilia society, ideally in collaboration with the haemophilia treatment centres. This needs to be a carefully considered decision for each individual as it is an irreversible one -off therapy. There is also a significant amount of monitoring required especially in the first year post gene therapy.
Variables And Unknowns
There are a significant number of variables and unknowns with hemophilia gene therapy, such as:
- Factor Expression: There was a wide variability in factor expression ranging from no increase to expression in the Supra normal (higher than expected) levels. For example, with the Licensed Factor 8 Gene Therapy, two years post-dosage, 15% of participants in the clinical trial had a factor 8 expression of greater than 40 IU/DL, 26% had a factor VIII level between 15 and 40 IU/DL, 35% had a factor 8 expression level between 5 and 15IU/DL, 10% had a factor expression between 3 and 5 IU/DL and 14% had a factor 8 expression of <3 IU/DL. After the first year post-dosage, factor 8 clotting factor use had decreased by 98%, annual bleed rate had decreased on average by 84% with the average annual bleed rate decreasing from 4.8 to 0.8.
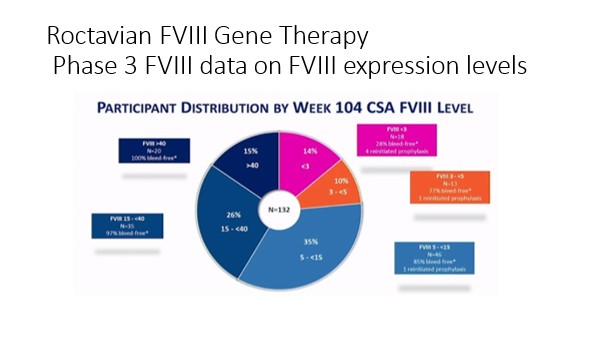
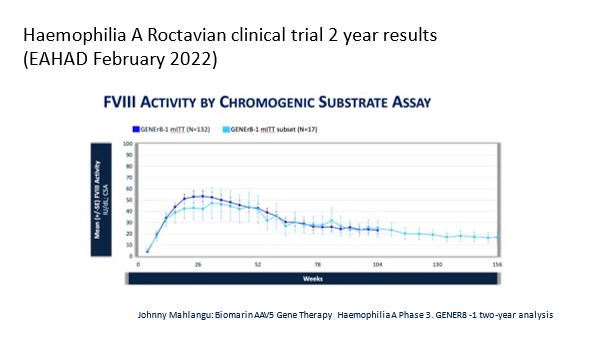
For the phase three data on the first factor IX gene therapy which is expected to be licensed soon, there was also a wide variability with factor expression although it was less so than with the factor 8 trial
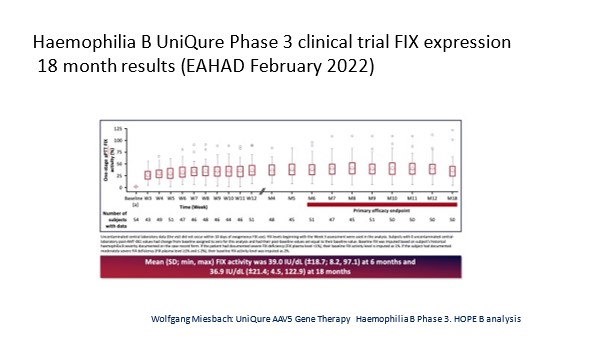
2. Predictability: We cannot predict which person with hemophilia will get a good response or not get a good response in terms of factor expression.
3. Durability: We cannot say how long significant factor expression will last. Current indications are that significant factor VIII expression may last for between five and eight years. For factor IX the durability looks more promising and may last for at least ten years and possibly for significantly longer.
4. Safety: Short-term safety concerns revolve primarily around the potential use of steroids as a result of liver inflammation which can happen when the gene therapy is delivered to the liver cells. For the licensed factor VIII gene therapy 86% of individuals had to take steroids for an average of seven months to deal with liver inflammation and for the phase three factor IX gene therapy 17% of individuals had to take steroids for an average of 80 days. Long term safety concerns revolve primarily around the potential for the gene therapy vector to be partially integrated into the person’s DNA. This integration could potentially result in an increased theoretical risk of cancer if the integration events occur in areas of the genome which are linked to cancer causation. To date there have been four cases of cancer in haemophilia gene therapy trials but following extensive investigation these have been found to be unrelated to the gene therapy. It must be remembered that people with hemophilia will also have a background “normal population” risk of cancer.
5. Affordability: Gene therapy may well be a very expensive therapy and if it has to be paid for on a one-off basis, this may have a very significant budget impact making it unaffordable for many countries. New payment models are currently being developed looking at outcome based annual payments for gene therapy.
