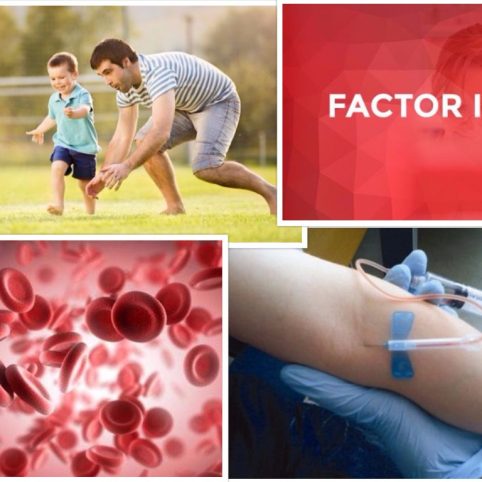
The Haemophilia Product Selection and Monitoring Advisory Board (HPSMAB) recently completed a tender process to choose a product for the treatment of people with factor IX deficiency. The group included the directors of the three Comprehensive Care Centres and representatives from the Irish Haemophilia Society (I.H.S.). The major selection criteria used in evaluating the products submitted for tender were safety, efficacy, quality, supply and cost. The product which scored highest in the tender process was an extended half-life factor IX (FIX) called Alprolix. Consequently, from the beginning of May, the FIX recombinant coagulation factor concentrate used in Ireland will be the extended half-life product Alprolix. This product has a half-life of approximately 77 hours, compared with a half-life of approximately 23 hours for the currently used product Benefix. It is anticipated that the vast majority of people with FIX deficiency, who are currently on prophylaxis will change from prophylaxis twice a week to prophylaxis once a week, while simultaneously maintaining higher trough levels and being provided with increased protection from bleeding. Ireland, to the best of our knowledge, is the first country in the world to switch all FIX patients to an extended half-life factor concentrate. Two extended half-life FIX concentrates came on the European market in late 2016, one of the products Alprolix has been on the US market for the best part of two years, so there is significant clinical experience with this product in the
US.