We have no current information on when and where people with haemophilia and other inherited bleeding disorders will be offered one of the licenced Covid-19 vaccines. Some work has been completed on guidance on giving the vaccine, bearing in mind that these vaccines must be injected by the intramuscular route.
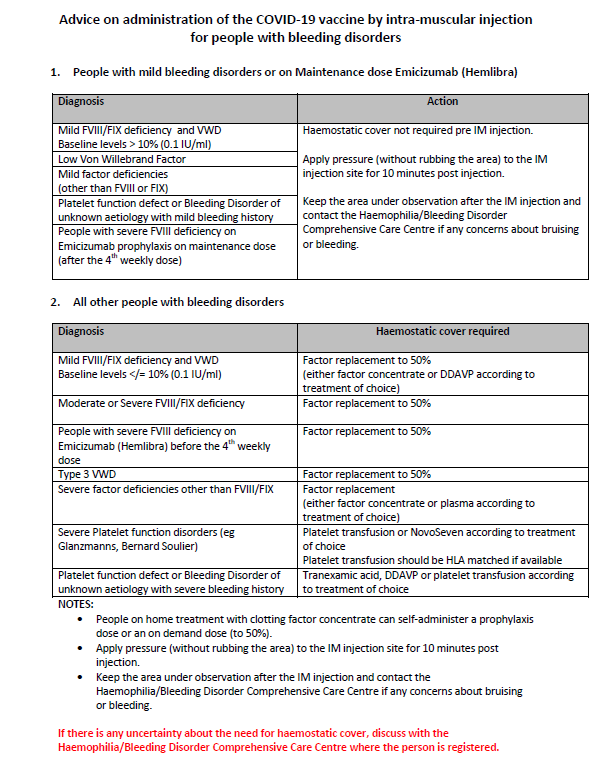
A guidance document for health care workers on vaccination of people with haemophilia, von Willebrands and other inherited bleeding disorders has just been produced by the National Coagulation Centre at St. James\’s Hospital. It is reproduced here, please download the PDF to you phone or device to bring with you to your vaccination appointment.
A guidance document has also been produced jointly by the World Federation of Hemophilia (WFH), European Association for Haemophilia and Allied Disorders (EAHAD), European Haemophilia Consortium (EHC), and U.S. National Hemophilia Foundation (NHF).
It is reproduced here:
World Federation of Hemophilia (WFH), European Association for Haemophilia and Allied Disorders (EAHAD), European Haemophilia Consortium (EHC), and U.S. National Hemophilia Foundation (NHF).
It is important that haemophilia treatment centres, in close collaboration with patient organizations, take action to inform people with bleeding disorders about the COVID-19 vaccines and contribute to an effective vaccination program.
1. People with bleeding disorders are not at greater risk of contracting COVID-19 or developing a severe form of the disease, so they are not considered a priority group for vaccination.
2. The vaccine should be administered intramuscularly. The smallest gauge needle available (25-27 gauge) should be used, if possible. Some vaccines must be administered using the accompanying needle–syringe combination, and so the use of an alternative needle may not be possible or desirable. Pressure should be applied to the site for at least 10 minutes post-injection to reduce bleeding and swelling. Additionally, self-inspection/palpation of the injection area several minutes and 2-4 hours later is recommended to ensure that there is no delayed hematoma. Discomfort in the arm felt for 1-2 days after injection should not be alarming unless it worsens and is accompanied by swelling. Any adverse events (e.g., haematoma, allergic reaction) should be reported to a haemophilia treatment centre.
3. Patients should contact their physician immediately or go to the nearest hospital emergency room right away if they experience an allergic reaction (fever, warmth, redness, itchy skin rash, shortness of breath, or swelling of the face or tongue) as it can be life-threatening. Patients with a history of allergic reactions to extended half-life clotting factor concentrates containing polyethylene glycol (PEG) should discuss vaccine choice with their physician because some vaccines contain PEG as an excipient.
4. Many individuals with bleeding disorders may not have ready access to haemostatic therapies prior to vaccination. In these cases, make efforts to access other clotting factors if possible. Alternatively, follow the instructions above making sure the smallest possible needle is used and maintain pressure for more than 10 minutes.
5. For patients with severe/moderate haemophilia, the injection should be given after a factor VIII (FVIII) or factor IX (FIX) injection. For patients with a basal FVIII or FIX level above 10%, no haemostatic precautions are required.
6. Patients on emicizumab (with or without an inhibitor) can be vaccinated by intramuscular injection at any time without haemostatic precautions and without receiving a dose of FVIII.
7. Patients with Type 1 or 2 Willebrand disease (VWD), depending on their baseline von Willebrand factor (VWF) ristocetin cofactor (RiCof) activity levels, should use therapies (i.e., DDAVP if available, tranexamic acid), in consultation with their haemophilia treatment centre. Patients with Type 3 VWD should be given a VWF-containing injection.
8. All rare bleeding disorder patients (including those with thrombocytopenia and/or platelet function disorders) should be vaccinated. Patients on anticoagulants should have prothrombin time testing performed within 72 hours prior to injection to determine international normalized ratio (INR); if results are stable and within the therapeutic range, they can be vaccinated intramuscularly.
9. There are no specific contraindications to vaccination related to complications of haemophilia and other bleeding disorders or their therapies. Immune tolerance, treatment of hepatitis C and HIV, and other conditions do not contraindicate vaccination.
10. Vaccination is not contraindicated for patients on immunosuppressive agents (cortisone, other immunosuppressive drugs).
11. Potential contraindications should be discussed individually with the physician because recommendations vary in different jurisdictions due to lack of data in special populations (e.g., pregnant or breastfeeding women).
12. The U.K. Medicines and Healthcare Products Regulatory Agency and the U.S. Centers for Disease Control and Prevention have advised caution in using the Pfizer/BioNTech vaccine in people with a history of significant allergic reactions. Specific recommendations for people with a history of allergic/anaphylactoid reactions can be found in the advisory published by each agency.
13. For patients in a clinical study, vaccination should be reported to the study investigators.
