First published in our regular e-Zine on April 9, 2021
Covid-19 Vaccination
The Society have been receiving a significant number of queries in relation to Covid-19 vaccination. Questions have been asked about prioritisation, where people will receive the vaccine, precautions to be taken when receiving an intramuscular injection and specific concerns about the Astra Zeneca vaccine. I will try to give some information on those points. We are clarifying some additional information with the Comprehensive care centres and we plan to send out a hard copy mailshot on Covid-19 next week together with an additional e-Zine.
Prioritisation
People with Haemophilia or other inherited bleeding disorders (PWBD) have not been separately identified as a priority group for vaccination. People with inherited bleeding disorders are not at higher risk of being infected with Covid-19 than the general public and if infected, they are not at higher risk of severe illness or death.
People with bleeding disorders will be vaccinated in the priority group which they will be part of normally as a result of other pre-existing conditions and/or age. PWBD over the age of 70 should already have been offered vaccine or should be offered vaccine very shortly. Vaccination after the over 70’s group will move on to Groups 4 (this group has started vaccination), then Group 5, 6 ,7 and following Group 7, vaccination will be by age starting with those aged 60-65 and then progressing to younger age groups.
Some PWBD may be eligible for vaccination in Group 4 if they meet any of the criteria listed below:
Group 4: People aged 16-69 with a medical condition that puts them at very high risk of severe disease and death
Cancer
All cancer patients actively receiving (and/or within 6 weeks of receiving) systemic therapy with cytotoxic chemotherapy, targeted therapy, monoclonal antibodies or immunotherapies and radical surgery or radiotherapy for lung or head and neck cancer.
All patients with advanced/metastatic cancers.
Chronic kidney disease
Chronic kidney disease, on dialysis, or eGFR <15 ml/min.
Chronic neurological disease or condition
Chronic neurological disease or condition with evolving ventilatory failure (requiring non-invasive ventilation), for example: motor neurone disease, spinal muscular atrophy.
Chronic respiratory disease
Chronic severe respiratory disease, for example: severe cystic fibrosis, severe COPD, severe pulmonary fibrosis.
Diabetes
Diabetes and HbA1C ≥58mmol/mol
Immunocompromised
Severe immunocompromise due to disease or treatment, for example:
– transplantation: – Listed for solid organ or haematopoietic stem cell transplant (HSCT) – Post solid organ transplant at any time – Post HSCT within 12 months
– genetic diseases: – APECED** – Inborn errors in the interferon pathway
– treatment: – included but not limited to Cyclophosphamide, Rituximab, Alemtuzumab, Cladribine or Ocrelizumab in the last 6 months
Inherited metabolic diseases*
Disorders of intermediary metabolism/at risk of acute decompensation, for example: Maple Syrup Urine Disease.
Intellectual disability*
Down Syndrome.
Obesity
BMI >40 Kg/m2.
Sickle cell disease
Some PWBD may be eligible for vaccination in Group 5 if they meet any of the criteria listed below:
Group 5: People aged 65-69 whose underlying condition puts them at a high risk of severe disease and death
Cancer
Haematological – within 1 year.
Haematological – within 1 – 5 years.
Non-haematological – within 1 year.
All other cancers on non-hormonal treatment.
Chronic heart (and vascular) disease
Chronic heart disease, for example: heart failure, hypertensive cardiac disease.
Chronic kidney disease
Chronic kidney disease with eGFR <30ml/min.
Chronic liver disease
Chronic liver disease, for example: cirrhosis or fibrosis.
Chronic neurological disease or condition
Chronic neurological disease or condition significantly compromising respiratory function and/or the ability to clear secretions, for example: Parkinson\’s disease, cerebral palsy.
Chronic respiratory disease
Other chronic respiratory disease, for example: stable cystic fibrosis, severe asthma (continuous or repeated use of systemic corticosteroids), moderate COPD.
Diabetes
All other diabetes (Type 1 and 2).
Immunocompromised
Immunocompromise due to disease or treatment, for example: high dose systemic steroids (as defined in Immunisation Guidelines for Ireland Chapter 3), persons living with HIV.
Inherited metabolic diseases*
Disorders of intermediary metabolism not fulfilling criteria for very high risk.
Intellectual disability*
Intellectual disability*** excluding Down Syndrome.
Obesity
BMI >35 Kg/m2.
Severe mental illness*
Severe mental illness, for example: schizophrenia, bipolar disorder, severe depression.
*additional or updated medical conditions
** APECED – autoimmune polyendocrinopathy candidiasis ecto- dermal dystrophy
*** WHO definition of intellectual disability as “impairments in adaptive, social, and intellectual functioning (IQ<70), requiring daily support, with onset in the developmental phase (<18 years)”
In this group, we would expect any PWBD between the age of 65 and 69 who was infected with HIV or Hepatitis C in the past to be included in addition to any person in this age group with Diabetes.
Some PWBD may be eligible for vaccination in Group 6 if they meet any of the criteria listed below:
Group 6: Other people aged 65-69 and key workers essential to the vaccine programme
These groups will be completed in parallel.
Other people aged 65-69
Rationale: At high risk of hospitalisation and death.
Ethical Principles: By protecting those at greatest risk of poor outcomes from the disease the principle of minimising harm is upheld.
Key workers essential to the vaccine programme
Rationale: Provide services essential to the vaccination programme
Some PWBD may be eligible for vaccination in Group 7 if they meet any of the criteria listed below:
Group 7: People aged 16-64 who have an underlying condition that puts them at high risk of severe disease and death
Cancer
Haematological – within 1 year.
Haematological – within 1 – 5 years.
Non-haematological – within 1 year.
All other cancers on non-hormonal treatment.
Chronic heart (and vascular) disease
Chronic heart disease, for example: heart failure, hypertensive cardiac disease.
Chronic kidney disease
Chronic kidney disease with eGFR <30ml/min.
Chronic liver disease
Chronic liver disease, for example: cirrhosis or fibrosis.
Chronic neurological disease or condition
Chronic neurological disease or condition significantly compromising respiratory function and/or the ability to clear secretions, for example: Parkinson\’s disease, cerebral palsy.
Chronic respiratory disease
Other chronic respiratory disease, for example: stable cystic fibrosis, severe asthma (continuous or repeated use of systemic corticosteroids), moderate COPD.
Diabetes
All other diabetes (Type 1 and 2).
Immunocompromised
Immunocompromise due to disease or treatment, for example: high dose systemic steroids (as defined in Immunisation Guidelines for Ireland Chapter 3), persons living with HIV.
Inherited metabolic diseases*
Disorders of intermediary metabolism not fulfilling criteria for very high risk.
Intellectual disability*
Intellectual disability*** excluding Down Syndrome.
Obesity
BMI >35 Kg/m2.
Severe mental illness*
Severe mental illness, for example: schizophrenia, bipolar disorder, severe depression.
In this group, we would expect any PWBD under the age of 65 who was infected with HIV or Hepatitis C in the past to be included in addition to any person in this age group with Diabetes.
Where will people with inherited bleeding disorders be vaccinated
Our present understanding is that PWBD will be vaccinated by their General Practitioner who should be aware of your medical history and the conditions which should place you in the appropriate group for vaccination.
It may be worthwhile contacting your GP, perhaps by e-mail to let them know that you should be on their vaccination list and drawing their attention to the specific group you are in for vaccination if in Groups 4, 5, 6 or 7. It may also be worthwhile letting them know that vaccination will not be carried out at the Haemophilia Treatment centres as your prioritisation for vaccination will depend on other co-morbidities and age and not on your underlying inherited bleeding disorder.
If an individual is not registered with a GP service, they should do so. The Social worker at the National Coagulation Centre, Gloria Rooney may be able to provide assistance with this if required (grooney@stjames.ie). We hope to have further information and clarification on these points from the Centre’s next week.
It may also be the case that some people will be directed to mass vaccination centres to receive their vaccine.
Vaccination timelines
It is clear from the constant updates from NPHET that the vaccine roll out dates and schedules are moving targets depending presently primarily on supply of vaccines.
Our current understanding is that GP’s will start vaccination of their patients in Group 4 on the week of April 12th and Group 7 on the week beginning May 3rd.
Precautions to be taken by people with inherited Bleeding disorders when receiving Covid-19 vaccination
Specific advice and precautions to be taken by those with inherited bleeding disorders when receiving the Covid-19 vaccine, which is an intramuscular injection have been previously set out by the National Coagulation Centre. They are as follows:
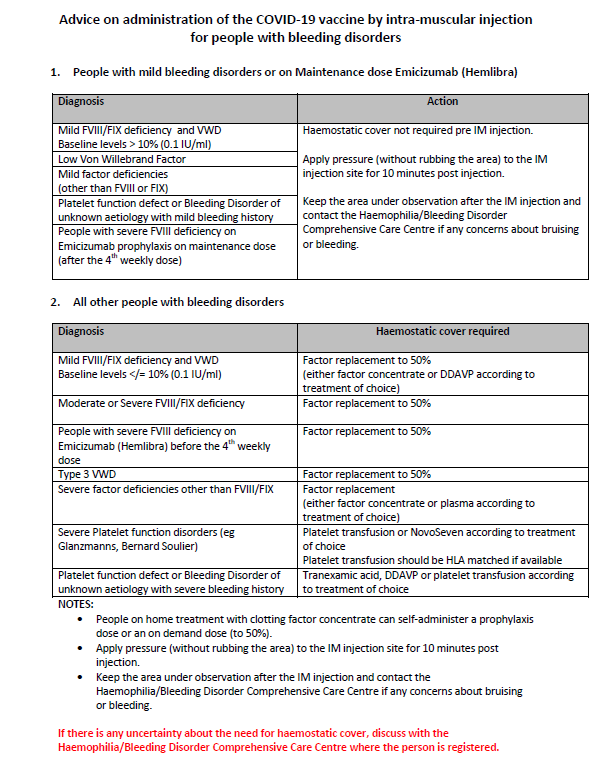
You can download the PDF from our website by clicking here.
Are there specific vaccinates which a PWBD should take or should avoid
The current advice remains that the benefits of any of 3 of the licenced vaccines in Ireland strongly outweigh any risks and that PWBD should take the first vaccine they are offered. There are no specific sub-groups within the population who are currently being advised to take a particular vaccine or not to take a particular vaccine.
Most of the recent concern regarding the safety profile of the vaccines have been focused on the Astra Zeneca vaccine. This vaccine has been linked to rare cases of a particular clotting problem. The information below is the latest available information from the National Coagulation Centre, posted on their website yesterday (April 8th). This follows the review of the safety of the Astra Zeneca vaccine earlier this week by the European Medicines Agency (EMA).
Astra Zeneca Covid-19 Vaccine (Vaxzevria)
Information for National Coagulation Centre (NCC) patients regarding the Astra Zeneca COVID19 vaccine
On 07/04/2021, the safety committee of the EMA have confirmed that the benefits of the Astra Zeneca COVID-19 vaccine in preventing severe Covid-19 disease continue to outweigh the risk of side effects. However, it has also been stated that a rare adverse event can occur within 14 days of receiving the Astra Zeneca Covid-19 vaccine which causes low platelet counts (a blood cell which clots the blood) and unusual blood clots, including in the veins of the head or abdomen. This rare side effect has been reported at a rate of approximately 1-2 cases per 100,000 vaccine doses given. These rare clotting events appear to be due to an unusual response from the immune system and Haematology specialists in Ireland are aware of the recommended blood tests and treatments if there is a suspicion of one of these rare cases.
Importantly, there is no evidence that the vaccine is linked to an increase in general blood clots (for example, deep vein thrombosis or pulmonary embolism). Also, there is no evidence that age, gender, past medical history including a past history of blood clotting or medications containing Oestrogen increase the risk for this rare complication.
People who have a past history of clotting (thrombosis) or who have a bleeding disorder are not at higher risk of this rare complication and people with these conditions are being advised to take whichever vaccine that is offered to them, in accordance with the National vaccine roll-out. It is not recommended to take any medications or other actions if you have previously had a history of clotting and you have had or are about to have the AstraZeneca vaccine.
Covid-19 vaccines, including the AstraZeneca vaccine, play an important role in containing this pandemic infection and in preventing COVID19 disease, which can be a severe condition with an increased risk of thrombosis. The National Immunisation Advisory Committee (NIAC) has recommended that the Astra Zeneca vaccine can be used as part of the vaccination programme in Ireland. At the moment, there is no change in the advice that this vaccine can be given to adults of all ages, though this advice is continuously under review.
NIAC has issued the following information for vaccine recipients:
Information for vaccine recipients
– COVID-19 Vaccine AstraZeneca is not associated with an increased overall risk of blood clotting disorders.
– There have been very rare cases of unusual blood clots accompanied by low levels of blood platelets (components that help blood to clot) after vaccination. The reported cases were almost all in women under 55 years of age. This may be due to vaccination of healthcare workers in this age group.
– Because COVID-19 can be so serious and is so widespread, the benefits of the vaccine far outweigh the risks of these very rare events.
– Seek prompt medical assistance and mention your recent vaccination if you get any of the following after receiving the COVID-19 Vaccine AstraZeneca:
• breathlessness,
• pain in the chest or stomach,
• swelling or coldness in a leg,
• severe or worsening headache or blurred vision after vaccination,
• persistent bleeding,
• multiple small bruises, reddish or purplish spots, or blood blisters under the skin
– Remember, some people will experience mild flu-like symptoms including headache, chills, fever and/or muscle aches. These are common side effects of any Covid-19 vaccine. These usually appear within a few hours and resolve within one or two days.
Advice for people who are on anticoagulants or have a bleeding disorder and are due to receive any COVID19 vaccine is available on the patient information tab of the NCC website
The European Medicines Agency advice referred to in the National Coagulation Centre statement from April 7th was as follows:
EMA confirms overall benefit-risk remains positive
EMA’s safety committee (PRAC) has concluded today that unusual blood clots with low blood platelets should be listed as very rare side effects of Vaxzevria (formerly COVID-19 Vaccine AstraZeneca).
In reaching its conclusion, the committee took into consideration all currently available evidence, including the advice from an ad hoc expert group. EMA is reminding healthcare professionals and people receiving the vaccine to remain aware of the possibility of very rare cases of blood clots combined with low levels of blood platelets occurring within 2 weeks of vaccination. So far, most of the cases reported have occurred in women under 60 years of age within 2 weeks of vaccination. Based on the currently available evidence, specific risk factors have not been confirmed.
People who have received the vaccine should seek medical assistance immediately if they develop symptoms of this combination of blood clots and low blood platelets (see below).
The PRAC noted that the blood clots occurred in veins in the brain (cerebral venous sinus thrombosis, CVST) and the abdomen (splanchnic vein thrombosis) and in arteries, together with low levels of blood platelets and sometimes bleeding.
The Committee carried out an in-depth review of 62 cases of cerebral venous sinus thrombosis and 24 cases of splanchnic vein thrombosis reported in the EU drug safety database (EudraVigilance) as of 22 March 2021, 18 of which were fatal.1 The cases came mainly from spontaneous reporting systems of the EEA and the UK, where around 25 million people had received the vaccine.
COVID-19 is associated with a risk of hospitalisation and death. The reported combination of blood clots and low blood platelets is very rare, and the overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects.
EMA’s scientific assessment underpins the safe and effective use of COVID-19 vaccines. Use of the vaccine during vaccination campaigns at national level will also take into account the pandemic situation and vaccine availability in the individual Member State.
One plausible explanation for the combination of blood clots and low blood platelets is an immune response, leading to a condition similar to one seen sometimes in patients treated with heparin (heparin induced thrombocytopenia, HIT). The PRAC has requested new studies and amendments to ongoing ones to provide more information and will take any further actions necessary.
Vaxzevria is one of four vaccines authorised in the EU for protecting against COVID-19. Studies show that it is effective at preventing the disease. It also reduces the risk of hospitalisation and deaths from COVID-19.
As for all vaccines, EMA will continue to monitor the vaccine’s safety and effectiveness and provide the public with the latest information.
Updated information on the Astra Zeneca vaccine
At the press conference following their advice, the EMA updated the numbers of cases of the rare clotting adverse event seen. In their formal announcement above, they had reviewed all the data from 25 million Astra Zeneca vaccinations in Europe up to March 22nd. 62 cases of the clot in the brain (cerebral venous sinus thrombosis, CVST) and 24 cases of clots in the abdomen the abdomen (splanchnic vein thrombosis) were reported or approximately 1 per 290,000 vaccinations. Updated numbers from April 4th in 34 million people vaccinated in Europe showed 169 reported cased of clots in the brain (cerebral venous sinus thrombosis, CVST) and 53 cases of clots in the abdomen (splanchnic vein thrombosis), approximately 1 per 153,000 vaccinations. Germany has reported cases at about 1 per 100,000 vaccinations. The information above from the NCC refers to 1-2 cases per 100,000. All these figures are risk estimates. There has been 1 case of this rare clotting side effect reported in Ireland, in a 40-year-old female who has recovered. (she did not have an inherited bleeding disorder).
The current advice is clearly that the benefit of the Astra Zeneca vaccine clearly outweighs the risk.
It is worth noting that serious side effects have been reported to date in approximately 1 per 100,000 Astra Zeneca vaccine recipients.
In Ireland 240,000 people have been infected with Covid 19 or almost 1 in 20 of the population.
In Ireland, 4,737 people have died of Covid 19 or 1 in 1,051 of the population.
Further information
We expect to receive some additional information early next week from the treatment centres and also a joint statement from the World Federation of Hemophilia (WFH) and the European Haemophilia Consortium (EHC). We will be sending out an update next week by mail and by e-Zine.
Brian O’Mahony,
Chief Executive
